This section assumes you have finished Tutorials Part 1 and Part 2
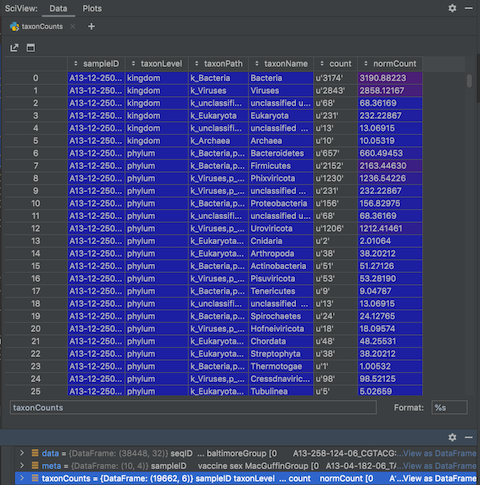
Unfiltered taxon counts
The taxonLevelCounts.tsv file is intended to make it quick and easy to compare virus hit counts across samples.
Let's look at the file by clicking on the 'View As DataFrame' link next to the 'taxonCounts' DataFrame:
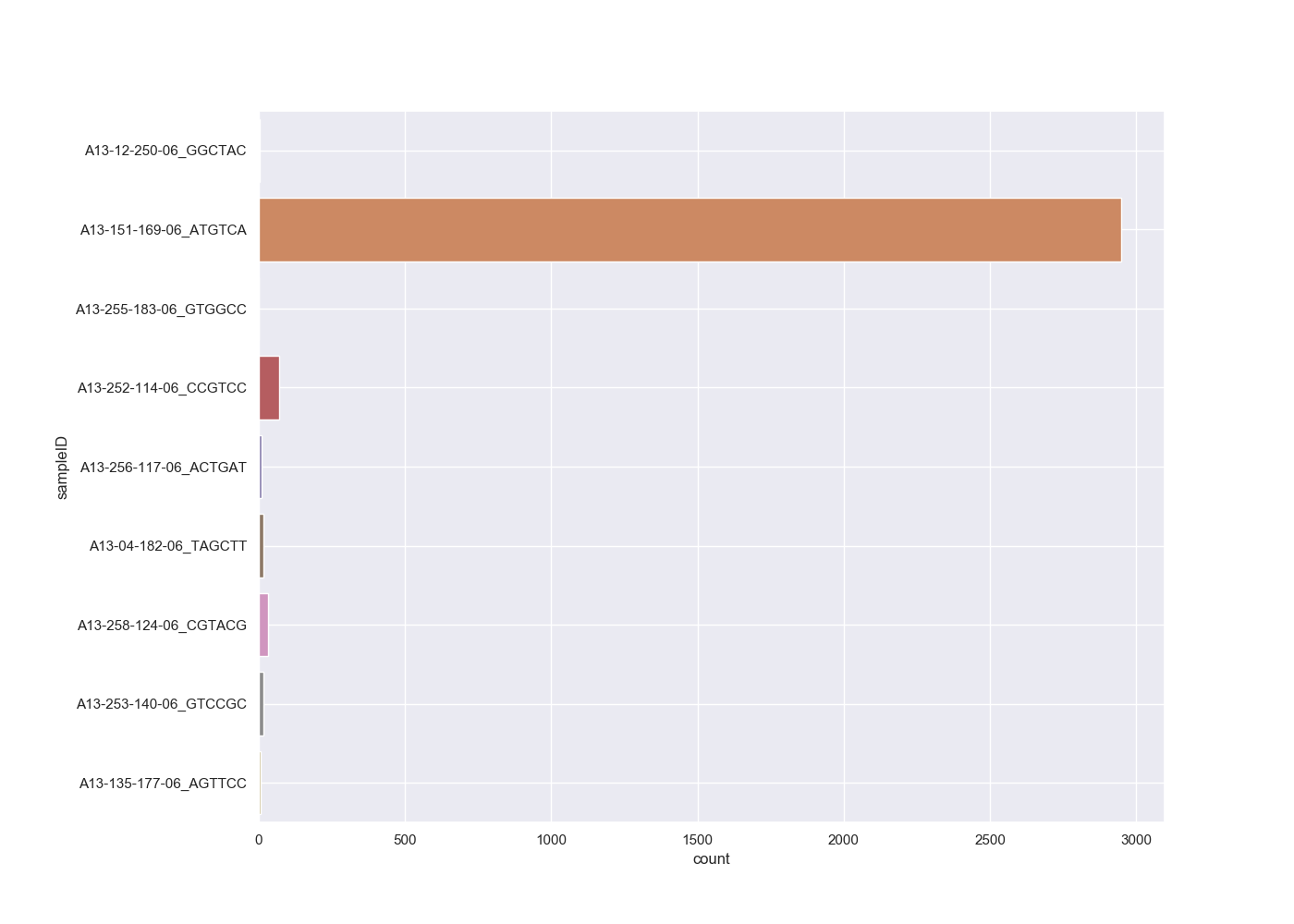
The file contains the counts and normalised counts for each taxonomic level, for each sample, based on the raw (unfiltered) hit counts. If we wanted to compare the number of Adenoviridae hits between each sample we could pull out the Adenoviridae counts and plot them:
# get adenoviridae counts
adenoCounts = taxonCounts[(taxonCounts.taxonLevel=='family') & (taxonCounts.taxonName=='Adenoviridae')]
# plot
sns.set_style("darkgrid")
sns.set_palette("colorblind")
sns.set(rc={'figure.figsize':(14,10)})
sns.barplot(x="count", y="sampleID", data=adenoCounts)
plt.subplots_adjust(left=0.2)
plt.grid(True)
plt.show()
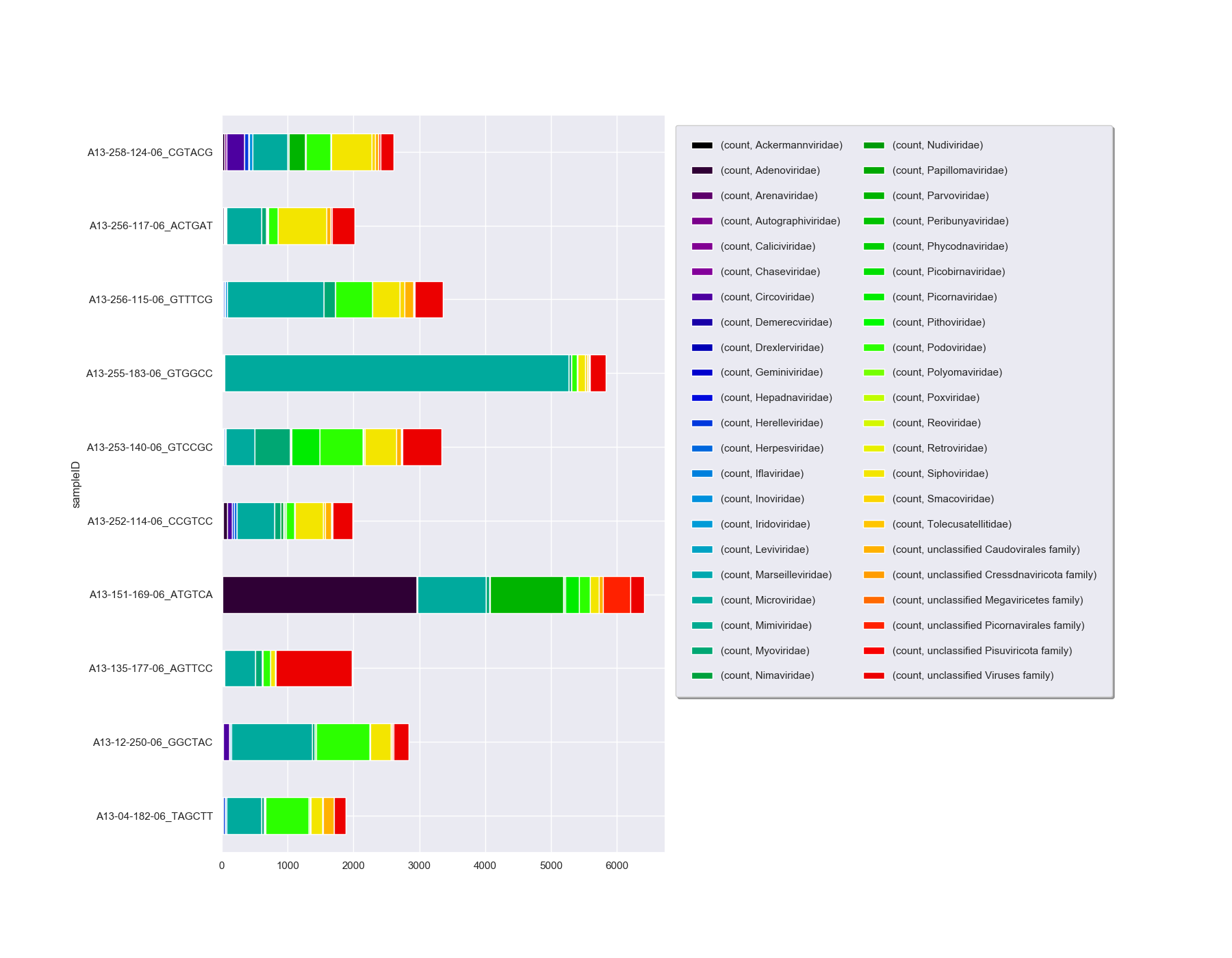
We can take this one step further and plot all the viral family normalised counts for each sample, in a stacked bar chart:
# get all viral family counts
viralCounts = taxonCounts[(taxonCounts.taxonLevel=='family') & (taxonCounts.taxonPath.str.contains('k_Viruses'))]
# plot
sns.set_style("darkgrid")
sns.set(rc={'figure.figsize':(18,14)})
colors = plt.cm.nipy_spectral(np.linspace(0, 1, 50))
viralCountsChartPivot=pd.pivot_table(viralCounts, index=['sampleID'], columns=['taxonName'], values=['count'], aggfunc='sum')
plt.figure();
viralCountsChartPivot.plot.barh(stacked=True,color=colors);
plt.subplots_adjust(left=0.18)
# Shink current axis by 50% to allow legend to fit nicely
ax = plt.gca()
box = ax.get_position()
ax.set_position([box.x0, box.y0, box.width * 0.5, box.height])
plt.legend(loc="upper left", bbox_to_anchor=(1.0, 1.0), borderaxespad=1,ncol=2, shadow=True, labelspacing=1.5, borderpad=1.5)
plt.show()
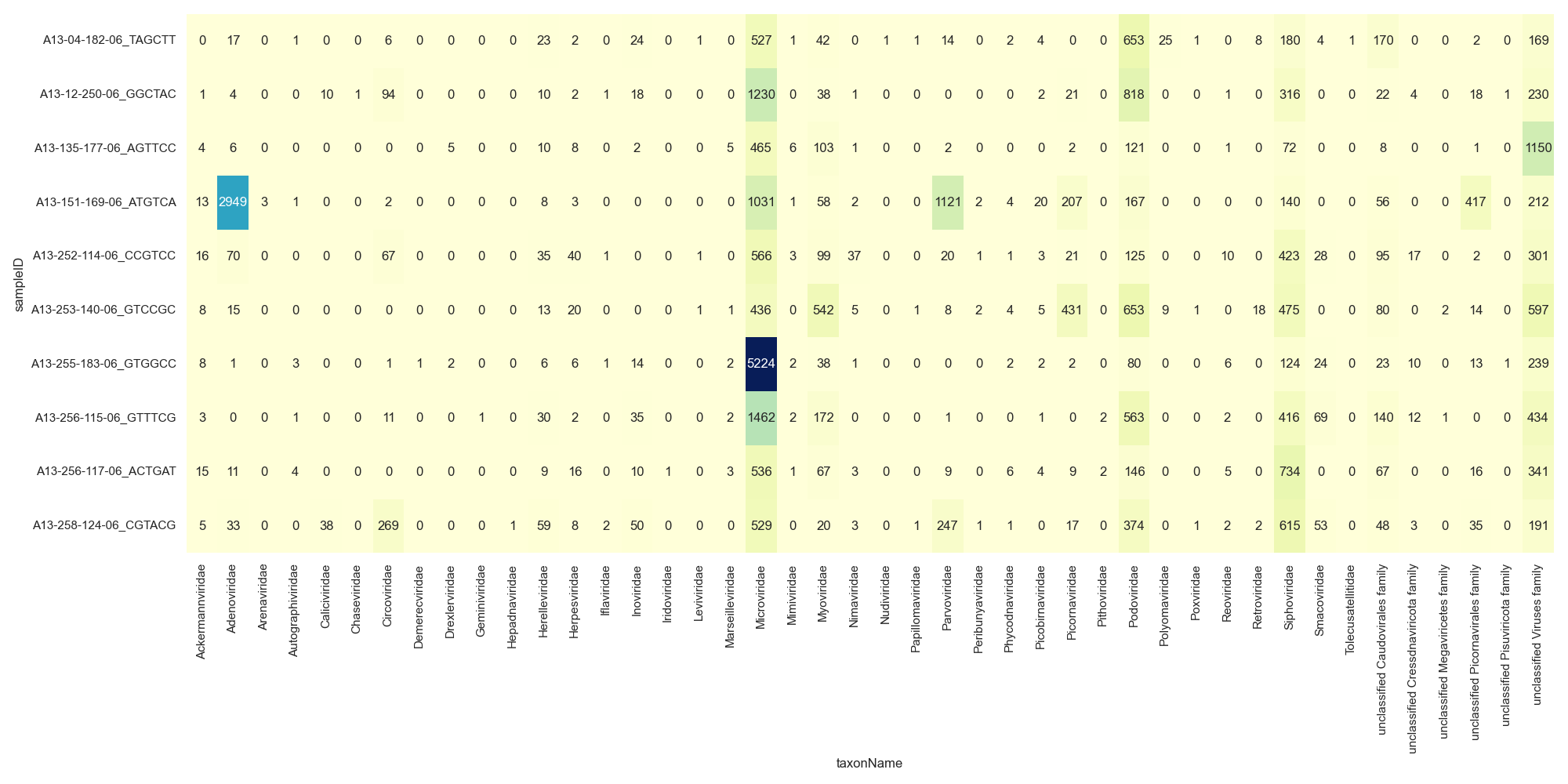
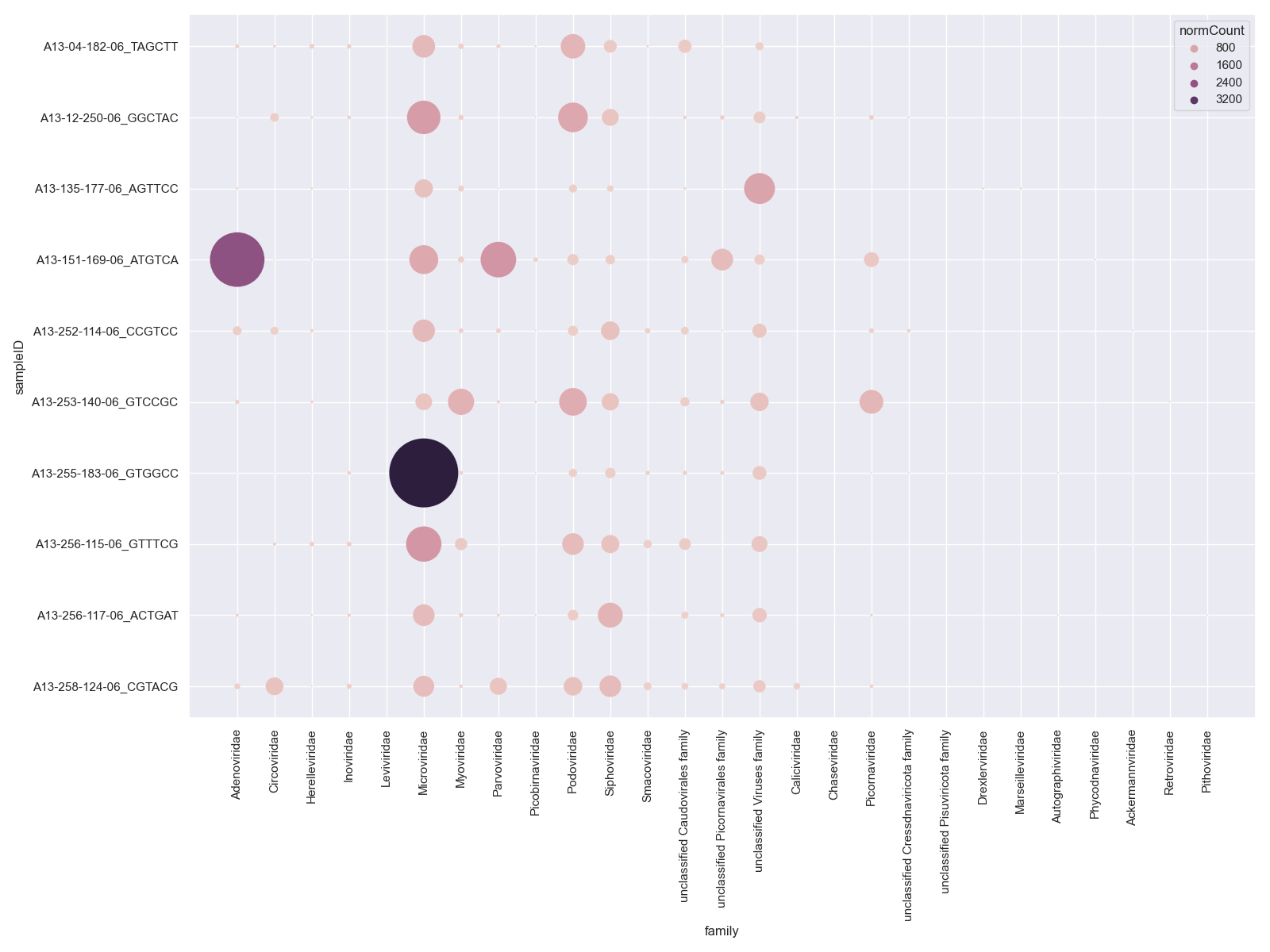
There are a couple of other options on how to display this data as you can see that the number of families is making this chart hard to interpret. You could look at either a Heatmap or Bubble Plot.
Heatmap
# get all viral family counts
viralFiltCounts = viralCounts.groupby(by=['sampleID','taxonName'], as_index=False)['count'].agg('sum')
#plot
sns.set_style("darkgrid")
sns.set(rc={'figure.figsize':(20,10)})
sns.heatmap(pd.crosstab([viralFiltCounts.sampleID], [viralFiltCounts.taxonName], values=viralFiltCounts['count'], aggfunc='sum', dropna=False).fillna(0),
cmap="YlGnBu", annot=True, cbar=False, fmt=".0f")
plt.xticks(rotation=90)
plt.tight_layout()
plt.show()
Bubble Plot
# get all viral family counts
viralFiltCounts = viralCounts.groupby(by=['sampleID','taxonName'], as_index=False)['count'].agg('sum')
#plot
sns.set_style("darkgrid")
sns.set(rc={'figure.figsize':(16,12)})
sns.scatterplot(x="taxonName", y="sampleID", data=viralFiltCounts, hue="count", s=viralFiltCounts['count'])
plt.xticks(rotation=90)
plt.tight_layout()
plt.show()
Generating taxon counts
The taxonLevelCounts.tsv file is convenient for comparing the raw counts,
but you will likely want to generate new counts from your filtered hits.
Recreate the above plot from the filtered hits by first summing the counts
or normalisedCounts, e.g. at the family level:
# Answer for "Challenge: Filter your raw viral hits to only keep protein hits with an evalue < 1e-10"
virusesFiltered = viruses[(viruses.alnType=='aa') & (viruses.evalue<1e-10)]
Then plot again and have included Heatmap or Bubble Plot options
Heatmap
sns.set_style("darkgrid")
sns.set(rc={'figure.figsize':(20,10)})
sns.heatmap(pd.crosstab([virusesFiltered.sampleID], [virusesFiltered.family], values=virusesFiltered.normCount, aggfunc='sum', dropna=False).fillna(0),
cmap="YlGnBu", annot=True, cbar=False, fmt=".0f")
plt.xticks(rotation=90)
plt.tight_layout()
plt.show()
Bubble Plot
sns.set_style("darkgrid")
sns.set(rc={'figure.figsize':(16,12)})
sns.scatterplot(x="family", y="sampleID", data=viralFiltCounts, hue="normCount", s=viralFiltCounts.normCount)
plt.xticks(rotation=90)
plt.tight_layout()
plt.show()
These count tables we will use for plotting and some statistical comparisons.
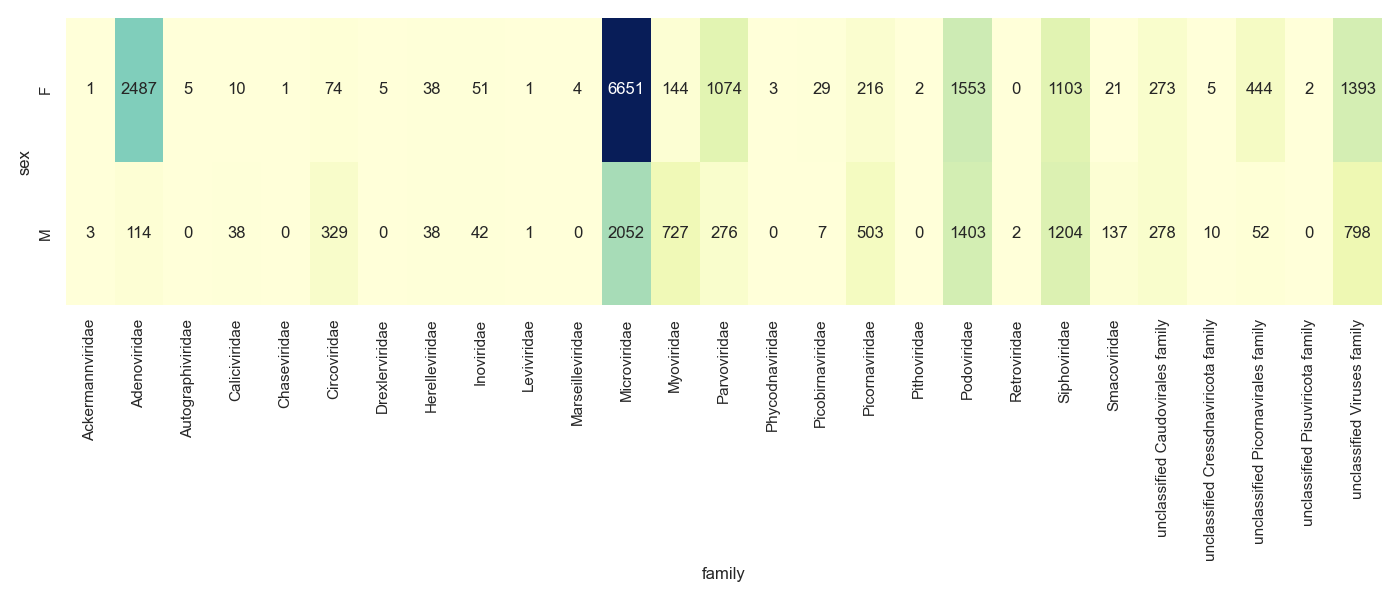
Challenge
Create either a stacked bar chart, heatmap or bubble plot of the viral families for the Male and Female monkeys
Visualising groups
We have a few viral families that are very prominent in our samples.
For the purposes of the tutorial we have a completely made up sample group category called MacGuffinGroup.
Let's see if there is a difference in viral loads according to our MacGuffinGroup groups.
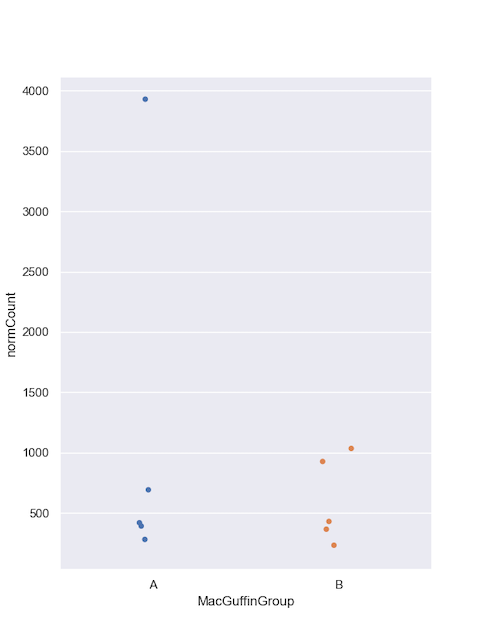
Collect sample counts for Microviridae.
Include the metadata group in group_by() so you can use it in the plot.
#filter
virusesFiltered = viruses[(viruses.family=='Microviridae') & (viruses.alnType=='aa') & (viruses.evalue<1e-10)]
#group by
microCounts = virusesFiltered.groupby(by=['MacGuffinGroup','family','sampleID'], as_index=False)['normCount'].agg('sum')
And plot. I like jitter plots but boxplots or violin plots might work better if you have hundreds of samples.
#plot
sns.set_style("darkgrid")
sns.set_palette("colorblind")
sns.set(rc={'figure.figsize':(6,8)})
sns.stripplot(x="MacGuffinGroup",
y="normCount",
data=microCounts, jitter=0.1)
plt.legend(bbox_to_anchor=(6.0,1), loc=0, borderaxespad=2,ncol=6, shadow=True, labelspacing=1.5, borderpad=1.5)
plt.show()
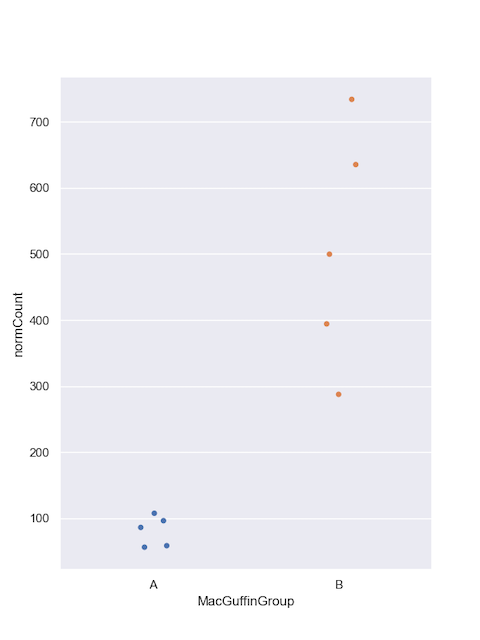
Let's do the same for Podoviridae.
#filter
virusesFiltered = viruses[(viruses.family=='Podoviridae') & (viruses.alnType=='aa') & (viruses.evalue<1e-10)]
#group by
podoCounts = virusesFiltered.groupby(by=['MacGuffinGroup','family','sampleID'], as_index=False)['normCount'].agg('sum')
#plot
sns.set_style("darkgrid")
sns.set_palette("colorblind")
sns.set(rc={'figure.figsize':(6,8)})
sns.stripplot(x="MacGuffinGroup",
y="normCount",
data=podoCounts, jitter=0.1)
plt.legend(bbox_to_anchor=(6.0,1), loc=0, borderaxespad=2,ncol=6, shadow=True, labelspacing=1.5, borderpad=1.5)
plt.show()
Challenge
Could gender be a good predictor of viral load for these families?
While the MacGuffinGroup looks promising for Podoviridae, we'll need to move on to Part 4: statistical tests to find out for sure.